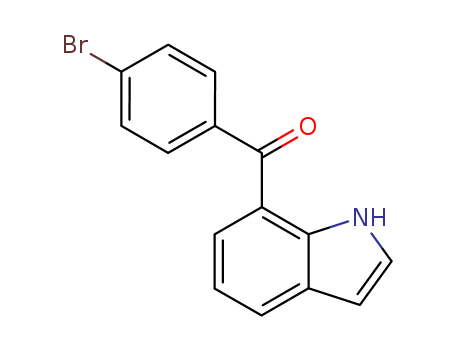
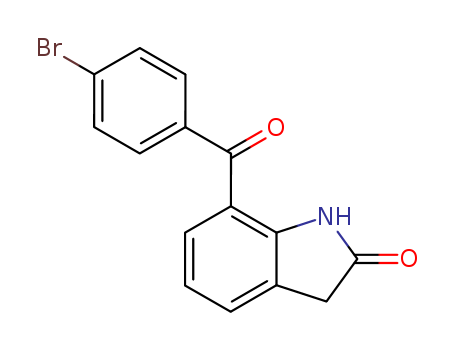
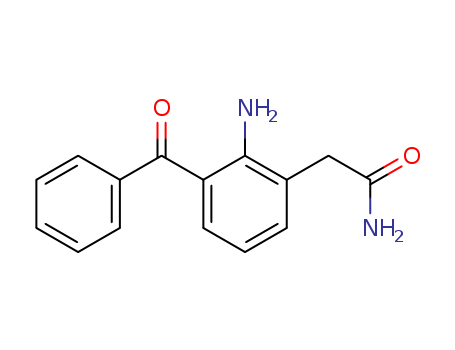
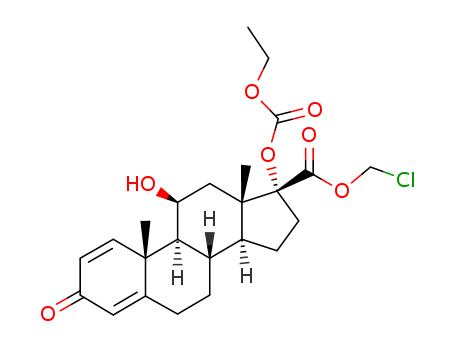
Quality Manufacturer Supply Wholesale Loteprednol Etabonate 82034-46-6 In Stock
- Molecular Formula:C24H31ClO7
- Molecular Weight:466.959
- Vapor Pressure:6.65E-17mmHg at 25°C
- Melting Point:220.5-223.5 °C
- Refractive Index:1.57
- Boiling Point:600.1 °C at 760 mmHg
- PKA:14.06±0.70(Predicted)
- Flash Point:316.7 °C
- PSA:99.13000
- Density:1.31 g/cm3
- LogP:3.91650
Loteprednol etabonate(Cas 82034-46-6) Usage and Manufacturer
Loteprednol etabonate is used to treat certain eye conditions due to inflammation or injury. It is also used after eye surgery. Loteprednol works by relieving symptoms such as swelling, redness, and itching. It belongs to a class of drugs known as corticosteroids. Shandong Kehui Pharmaceutical Co., Ltd. is a modern high-tech pharmaceutical company that strictly complies with GMP requirements and produces high standards. The company was established in May 2017 and is located in the Yellow River Delta Medicine Valley Industrial Park, Gaoqing County, Zibo City. At present, a professional production workshop, strict quality management and inspection system, and a complete EHS management system have been built. The company's main business scope includes the production and sales of APIs and intermediates, pharmaceutical excipients, eye drops, and tablets. Shandong Kehui Pharmaceutical Co., Ltd. has built three production lines for calcium antagonists, ophthalmic raw materials, and hormones in the first phase. It has technical advantages in the R&D and production of ophthalmic drugs and calcium antagonist antihypertensive drugs, and realizes independent research and development of key technologies. It has the characteristics of small scale and large market, small output and high output value. At present, it can produce 30 tons of calcium antagonist raw materials per year, and 1 tons of ophthalmic raw materials such as bromfenac sodium, nepafenac, pranoprofen, loteprednol, and besifloxacin hydrochloride. Shandong Kehui Pharmaceutical Co., Ltd. has formed a unique product chain from research, production to sales. The sales are based on the domestic market and face the high-end regulatory market. The products are sold to Europe, the United States, Japan, South Korea, India and other countries.
InChI:InChI=1/C24H31ClO7/c1-4-30-21(29)32-24(20(28)31-13-25)10-8-17-16-6-5-14-11-15(26)7-9-22(14,2)19(16)18(27)12-23(17,24)3/h7,9,11,16-19,27H,4-6,8,10,12-13H2,1-3H3/t16-,17-,18-,19+,22-,23-,24-/m0/s1
82034-46-6 Relevant articles
Loteprednol etabonate 0.5% versus prednisolone acetate 1.0% for the treatment of inflammation after cataract surgery
Stephen S. Lane MD , Edward J. Holland MD
, Journal of Cataract & Refractive Surgery Volume 39, Issue 2, February 2013, Pages 168-173
The study enrolled 88 patients (46 loteprednol etabonate, 42 prednisolone acetate). Equivalency was achieved between the 2 treatment groups with no significant differences throughout the 3-week follow-up. There was less fluctuation in IOP assessments in patients treated with loteprednol etabonate than in patients treated with prednisolone acetate, in particular 1 day and 3 days postoperatively. The results indicate that equivalent control of inflammation can be obtained through treatment with loteprednol etabonate or prednisolone acetate after cataract surgery. In addition, treatment with loteprednol etabonate may result in less IOP fluctuation.
Steroid-induced ocular hypertension with loteprednol etabonate 0.2%—A case report
Edeline Lu O.D. a , Lane T. Fujimoto O.D. a b , Paul A. Vejabul O.D. a b , Russell L. Jew O.D. a b
, Optometry - Journal of the American Optometric Association Volume 82, Issue 7, July 2011, Pages 413-420
The aim of this study was to present a case of a patient who showed a significant increase in intraocular pressure (IOP) with topical administration of loteprednol etabonate 0.2%. Although loteprednol etabonate has been shown to have a minimal effect on IOP in most patients, close follow-up is necessary whenever therapy is initiated.
Comparison between Betamethasone, Fluorometholone and Loteprednol Etabonate on intraocular pressure in patients after keratorefractive surgery
Saeed Shokoohi-Rad , Ramin Daneshvar , Mahsa Jafarian-Shahri , Parisa Rajaee
, Journal of Current Ophthalmology Volume 30, Issue 2, June 2018, Pages 130-135
The aim of this study was to compare the ocular hypertensive effect of the commercially available Betamethasone, Fluorometholone in Iran and Loteprednol Etabonate in patients undergoing keratorefractive surgery. Loteprednol and Fluorometholone were associated with the most and least increase in IOP, respectively. The highest pressures were detected 4 weeks after surgery in the Betamethasone and Loteprednol groups and 6 weeks after surgery in the Fluorometholone group. Fluorometholone was the safest among the three examined steroid drops in terms of IOP rise.
82034-46-6 Upstream products
-
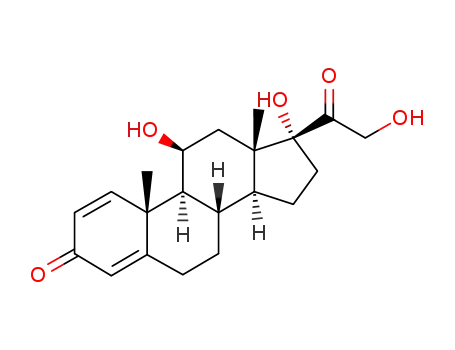
50-24-8
prednisolon
-
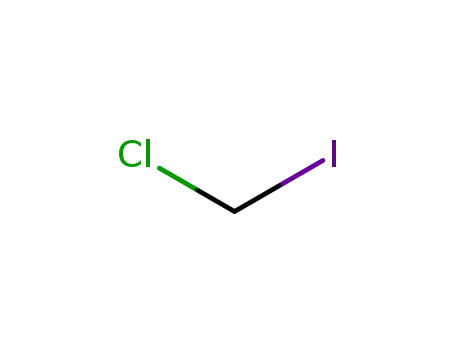
593-71-5
Chloroiodomethane
-
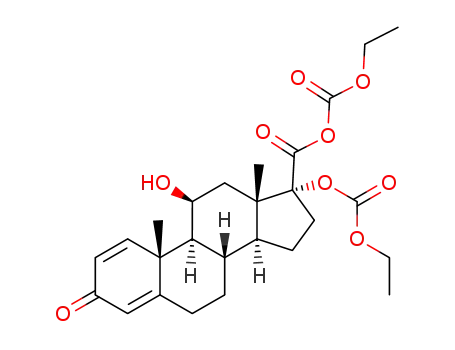
133991-62-5
17α-[(ethoxycarbonyl)oxy]-11β-hydroxy-3-oxoandrosta-1,4-diene-17β-carboxylic ethoxycarboxylic anhydride
-
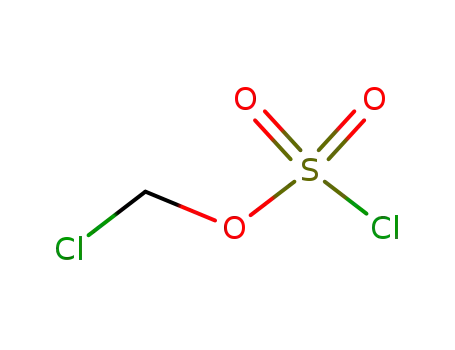
49715-04-0
chlorosulfuric acid chloromethyl ester
82034-46-6 Downstream products
-
37927-29-0
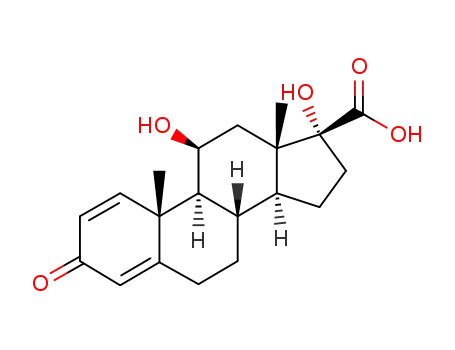
(1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-2,15-dimethyl-5-oxotetracyclo[8.7.0 02,7. 011,15]heptadeca-3,6-diene-14-carboxylic acid
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego